I could not have said it better and PEW’s conclusion is spot on:
Current poultry policies and regulations treat all Salmonella serotypes and strains as if they pose equal risks to people, despite science showing this is not the case. Food safety interventions on farms, such as vaccines targeting harmful serotypes, have likely contributed to meaningful reductions in product contamination and human illness. FSIS should revise its rules to focus more intensively on the serotypes causing more frequent or severe infections, and implement other policies to prompt poultry operations to adopt on-farm practices that control these hazards. Such a risk-based approach would help prevent illnesses and outbreaks linked to poultry products and drive down the nation’s unacceptably high number of Salmonella illnesses.
Here is the story visually:
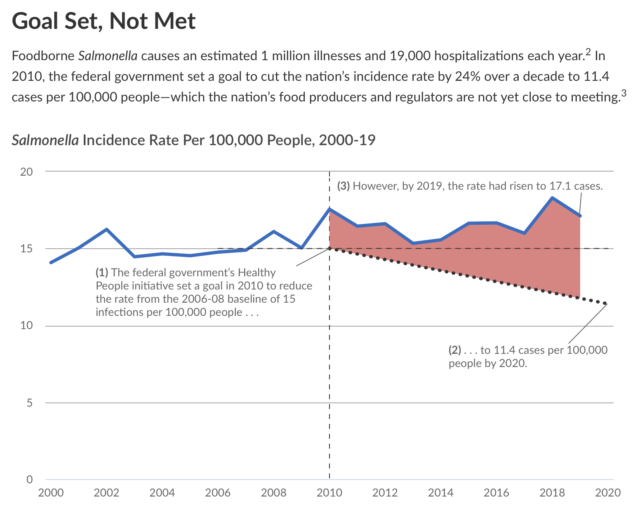





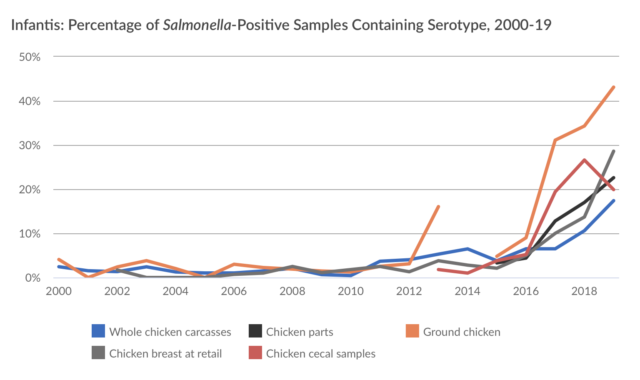


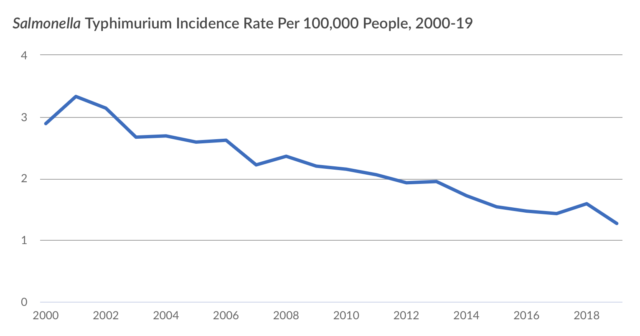
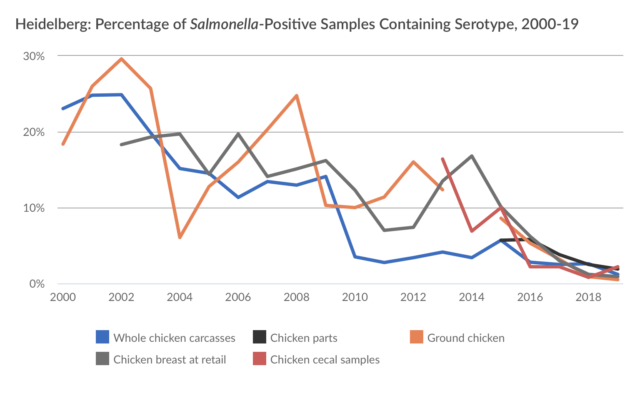

I agree with PEW’s concern, as I recently noted in a letter to USDA/FSIS:
Marler Clark LLP, PS submits this letter requesting a definitive and prompt response to the above-referenced docket, Docket No. FSIS-2020-0007; Document ID FSIS-2020-0007-0001 – Petition for an Interpretive Rule declaring ‘Outbreak’ Serotypes of Salmonella enterica subspecies enterica to be Adulterants Within the Meanings of 21 U.S.C. § 601(m)(1) and 21 U.S.C. § 453(g)(1) (hereinafter “Salmonella Petition” or “Petition”).
As stated in our November 12, 2021, letter, nearly two years have elapsed since the submission of our SalmonellaPetition on behalf of Rick Schiller, Steven Romes, the Porter family, Food & Water Watch, Consumer Federation of America, and Consumer Reports, requesting that FSIS declare the following “Outbreak Serotypes” to be per seadulterants in meat and poultry products:
Salmonella Agona, Anatum, Berta, Blockely, Braenderup, Derby, Dublin, Enteritidis, Hadar, Heidelberg, I 4,[5],12:i:-, Infantis, Javiana, Litchfield, Mbandaka, Mississippi, Montevideo, Muenchen, Newport, Oranienburg, Panama, Poona, Reading, Saintpaul, Sandiego, Schwarzengrund, Senftenberg, Stanley, Thompson, Typhi, and Typhimurium[1].
Since then—although FSIS is required by the Administrative Procedure Act[2] and the courts[3] to, at the very least, respond to the merits of a petition for rulemaking—we have yet to receive a clear response as to when or how FSIS intends to address our Petition.
We write to supplement the November 12, 2021, letter with new research. In their newly published review paper[4], O’Bryan et al. explain that, although the current USDA qualitative performance standards have lowered the prevalence of Salmonella found on raw poultry products, progress has stalled on lowering the cases of salmonellosis associated with poultry. They write, “the incidence of salmonellosis (most recent data indicates 15.3 cases per 100,000) is still well above CDC’s Healthy People 2030 objective of 11.5 cases per 100,0000 population and has not experienced substantial reductions in the last two decades, despite the prevalence data exhibiting a steady decline[5].”
The authors list several reasons for this standstill, many of which are further detailed in the Marler Clark Petition: there are numerous means for birds to be contaminated by Salmonella during the production cycle, especially within the processing environment (e.g., during picking, evisceration, and immersion in the chiller tanks); there is a high prevalence of unsafe behaviors[6] (e.g., undercooking and poor handwashing technique) when cooking poultry, as well as a lack of consumer education; and the current routine monitoring of Salmonella occurrence in poultry processing is seriously lacking. To remedy the latter, they suggest that “the food and poultry industry … undertake a more proactive stance” and develop “a much more complete evaluation of Salmonella population concentrations on a carcass testing positive for Salmonella[7].”
Despite its confirmation of the continuing food safety crisis caused by the failure of the USDA to adequately address the contamination of poultry with Salmonella outbreak strains, the authors still make a glaring error in critiquing the position taken by the Petition. Specifically, the authors assert “that the inherent presence of Salmonella in poultry means it cannot be an ‘added substance’ and it therefore should not be considered an adulterant[8].” The FMIA unambiguously defines an adulteration standard for substances that are “not added” and a different standard for those that are “added[9].” Further, the USDA has itself repeatedly rejected the argument that a bacterial pathogen cannot be deemed an adulterant “because the organism may be inherent in raw meat and poultry when produced under current technology[10].” Indeed, in rejecting this argument, the USDA cited to its own court victory in defending its decision to treat E. coli O157:H7 as an adulterant, despite vociferous industry objections[11]. Thus, having rejected these arguments before, the USDA must do so again. And, finally, the USDA would lack the authority to seek the recall of Salmonella-contaminated ground beef and poultry—recalls that it has done repeatedly—if there was no basis under the FMIA to treat Salmonella, in those instances, as an adulterant.
The other arguments made against the Petition are no better than the inherency one[12]. Although there are no serotype-specific interventions, each of the 31 Outbreak Serotypes of Salmonella we seek be deemed adulterants has a demonstrable history associated with either an illness outbreak or a product recall and are proven to be injurious to human health. Altogether, these 31 Outbreak Serotypes currently account for the greatest number of Salmonella illnesses[13]. As Salmonella evolve, however, serotypes of public concern could be added (as was the case with the “Big Six” non-O157 strains of STEC E. coli) or subtracted from a variable list of outbreak serotypes.
Each year, CDC’s Interagency Food Safety Analytics Collaboration (IFSAC) generates a report that uses outbreak data to produce annual estimates for foods responsible for foodborne illnesses caused by four pathogens, including Salmonella[14]. In its latest publication[15], IFSAC, using data from 1,532 foodborne disease outbreaks that occurred from 1998 through 2019, reported that chicken is responsible for 16.8% of all Salmonella illnesses, making it the single most important source of Salmonella illnesses of any food category. Chicken and turkey, taken together, account for over 23% of those illnesses.
The percentage of Salmonella illnesses associated with poultry has increased year-over-year. In 2015[16], IFSAC reported that chicken was the second largest source of Salmonella illnesses, responsible for 11.8% of Salmonella illnesses. The following year, IFSAC reported that chicken was responsible for 12.7% of Salmonella illnesses[17]. In 2017, chicken was linked to 14% of foodborne illnesses attributed to Salmonella[18]. The following year, in 2018[19], chicken became the number one source of Salmonella illnesses (dethroning seeded vegetables), responsible for 14.3% of illnesses.
In its October 19, 2021, press release, the USDA announced that “it is mobilizing a stronger, and more comprehensive effort to reduce Salmonella illnesses associated with poultry products,” with the goal of “mov[ing] closer to the national target of a 25% reduction in Salmonella illnesses[20].” In doing so, “FSIS will focus on the Salmonellaserotypes and the virulence factors that pose the greatest public health risk.” Although we support the USDA’s efforts, we do not consider this announcement to be responsive to our Petition.
To protect the public, FSIS needs to acknowledge that certain Salmonella serotypes pose an unacceptable risk to consumers and make rules to keep adulterated products contaminated by these serotypes off the shelves. Accordingly, we again urge you to grant our Petition. If we do not receive a definitive response within 140 days, we will assume that you have denied our petition and proceed with judicial remedies.
_______________________
[1] Thirty of these 31 serotypes are from the Centers for Disease Control and Prevention’s (CDC) Salmonella Atlas, which contains 42 years of laboratory-confirmed research. See Salmonella Atlas at https://www.cdc.gov/salmonella/reportspubs/salmonella-atlas/serotype-reports.html. The only exception, Salmonella Dublin, was added to Petitioners’ list because it is a serotype of increasing public health concern that was recently involved in a foodborne illness outbreak linked to ground beef.
[2] In addition to 5 USC § 553(e)’s requirement that each agency “shall give an interested person the right to petition for the issuance, amendment, or repeal of a rule,” the Administrative Procedure Act also requires agencies to provide “prompt notice…of the denial in whole or in part of a written application, petition, or other request of an interested person made in connection with any agency proceeding,”5 USC §555(e).
[3] Horne v. USDA, 494 Fed. Appx. 774 (9th Cir. 2012) (“USDA responded to the Hornes’ rulemaking petition—as it must under the Administrative Procedure Act”); WWHT, Inc. v. F.C.C., 656 F.2d 807, 813 (D.C. Cir. 1981) (“an agency must receive and respond to petitions for rulemaking”); Nat’l Parks Conserv. Ass’n v. Interior, 794 F.Supp.2d 39, 44-45 (D.D.C. 2011) (“[A]n agency ‘is required to at least definitively respond to . . . [a] petition—that is, to either deny or grant the petition.’”); Families for Freedom v. Napolitano, 628 F.Supp.2d 535,540 (S.D.N.Y 2009) (concluding the same and noting “DHS conceded this point at oral argument”); but see Brown v. FBI, 793 F.Supp.2d 368, 375 (D.C. Cir. 2011) (observing, in the context of reviewing petitioner’s standing, that “the APA is less than crystal-clear on plaintiff’s statutory right to a response,” though simultaneously citing WWHT, “an agency must receive and respond”). See also Richard J. Pierce, Administrative Law Treatise 517 (5th ed. 2013) (“At a minimum, the right to petition for rulemaking entitles a petitioning party to a response to the merits of the petition.”).
[4] O’Bryan, C. A., S. C. Ricke, J. A. Marcy (2021). Public health impact of Salmonella spp. on raw poultry: Current concepts and future prospects in the United States. Food Control. 132:108539. https://doi.org/10.1016/j.foodcont.2021.108539
[5] Id.
[6] See, e.g., Kirchner, M. K., R. M. Goulter, B. J. Chapman, J. Clayton, L. Jaykus. (2021). Cross-Contamination on Atypical Surfaces and Venues in Food Service Environments. J Food Prot. 84(7):1239-51.Verrill, L., et al. (2021). Hand Washing Observations in Fast-Food and Full-Service Restaurants: Results from the 2014 U.S. Food and Drug Administration Retail Food Risk Factors Study. J Food Prot. 84(6):1016-22.Cardoso, M. J., V. Ferreira, M. Truninger, R. Maia, P. Teixeira. (2021). Cross-contamination events of Campylobacter spp. in domestic kitchens associated with consumer handling practices of raw poultry. Int J Food Microbiol. 338:108984.Mihalache O. A., D. Borda, C. Neagu, P. Teixeira, S. Langsrud, A. I. Nicolau. (2021). Efficacy of Removing Bacteria and Organic Dirt from Hands—A Study Based on Bioluminescence Measurements for Evaluation of Hand Hygiene When Cooking. Int J Environ Res Public Health. 18(16): 8828.Cohen, N. L., R. B. Olson. (2016). Compliance With Recommended Food Safety Practices in Television Cooking Shows. J Nutr Educ Behav. 48(10): 730-34.Oscar, T. P. (2013). Initial contamination of chicken parts with Salmonella at retail and cross-contamination of cooked chicken with Salmonella from raw chicken during meal preparation. J Food Prot. 76(1):33-9.Carrasco, E., A. Morales-Rueda, R. M. Garcia-Gimeno. (2012). Cross-contamination and recontamination by Salmonella in foods: A review. Food Res Int. 45(2):545-56.
[7] O’Bryan, C. A., S. C. Ricke, J. A. Marcy (2021). Public health impact of Salmonella spp. on raw poultry: Current concepts and future prospects in the United States. Food Control. 132:108539. https://doi.org/10.1016/j.foodcont.2021.108539
[8] Id.
[9] 21 U.S.C. § 601(m)(1); see also Young v. Community Nutrition Institute, 476 U.S. 974, 977 (1986) (addressing regulatory requirements for “adulterants,” like aflatoxins, that are “not added,” and noting the requirements are more strict for substances that are added)
[10] USDA, Recent Developments Regarding Beef Products Contaminated with E. coli O157:H7, 65 Fed. Reg. 6881, 6884 (Feb. 11, 2000)
[11] Id. (citing Texas Food Industry Ass’n v. Espy, 870 F. Supp. 143 (WD Tex. 1994)).
[12] “There are no serotype-specific interventions for Salmonella, and there are no practical or reliable ways to rapidly identify serotypes in-plant, much less to have a high degree of confidence that all serotypes present in a flock have been identified. Furthermore, it would also be impractical to declare 31 serotypes of Salmonella as adulterants while ignoring the other roughly 2,500 serotypes. The evolution in Salmonella over time would suggest that this would not remain a static list of Salmonella serovars but would likely change the number of distinctive strains for a given serotype over time and the pathogenesis characteristics in different serovars could evolve over time.”
[13] We explain this point further on page 14 of the Petition: “According to a recent report by the National Advisory Committee on Microbiological Criteria for Foods (NACMCF), highly virulent strains are virtually indistinguishable from non-virulent ones because ‘virulence markers for gastroenteritis are not serotype specific.’ Nevertheless, certain serotypes of NTS (Heidelberg, Sandiego, Schwarzengrund, Panama, Poona, Oranienburg) are ‘more likely to escape the gastrointestinal tract and cause systemic disease.’ Moreover, according to the report, a few serotypes are ‘consistently associated with the greatest incidence of human disease,’ including Salmonella enterica serotypes Newport, Enteritidis, Javiana, Typhimurium, Infantis, Muenchen, and I 4,[5],12:i:-. These serotypes (and others) are thoroughly documented in CDC’s Salmonella Atlas and are readily identifiable using Whole Genome Sequencing (WGS).”
[14] See IFSAC’s reports at https://www.cdc.gov/foodsafety/ifsac/annual-reports.html
[15] “Foodborne illness source attribution estimates for 2019 for Salmonella, Escherichia coli O157, Listeria monocytogenes, and Campylobacter using multi-year outbreak surveillance data, United States.” IFSAC, October 2021. https://www.cdc.gov/foodsafety/ifsac/pdf/P19-2019-report-TriAgency-508.pdf
[16] “Foodborne illness source attribution estimates for 2015 for Salmonella, Escherichia coli O157, Listeria monocytogenes, and Campylobacter using multi-year outbreak surveillance data, United States.” IFSAC, November 2018. https://www.cdc.gov/foodsafety/ifsac/pdf/P19-2015-report-TriAgency-508.pdf
[17] “Foodborne illness source attribution estimates for 2016 for Salmonella, Escherichia coli O157, Listeria monocytogenes, and Campylobacter using multi-year outbreak surveillance data, United States.” IFSAC, November 2018. https://www.cdc.gov/foodsafety/ifsac/pdf/P19-2016-report-TriAgency-508.pdf
[18] “Foodborne illness source attribution estimates for 2017 for Salmonella, Escherichia coli O157, Listeria monocytogenes, and Campylobacter using multi-year outbreak surveillance data, United States.” IFSAC, September 2019. https://www.cdc.gov/foodsafety/ifsac/pdf/P19-2017-report-TriAgency-508-revised.pdf
[19] “Foodborne illness source attribution estimates for 2018 for Salmonella, Escherichia coli O157, Listeria monocytogenes, and Campylobacter using multi-year outbreak surveillance data, United States.” IFSAC, December 2020. https://www.cdc.gov/foodsafety/ifsac/pdf/P19-2018-report-TriAgency-508.pdf
[20] “USDA Launches New Effort to Reduce Salmonella Illnesses Linked to Poultry.” USDA, October 19, 2021. https://www.usda.gov/media/press-releases/2021/10/19/usda-launches-new-effort-reduce-salmonella-illnesses-linked-poultry